By Ellen Kamhi PhD, RN, AHG, AHN-BC and Eugene Zampieron, ND, AHG www.drznaturally.com
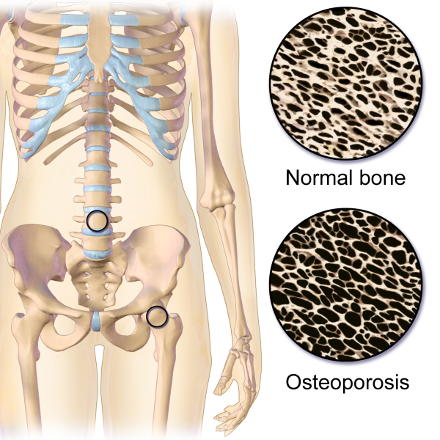
Osteoporosis is a disease of the skeletal system, which is characterized by deterioration of bone tissue, along with a decrease in bone mass. It can strike anyone at any age, although it is most prevalent in Caucasian and Asian, small boned woman over 50 years old. Osteoporosis is recognized as a major public health issue. Over 10 million Americans are afflicted, with 34 million more who may already be exhibiting signs of low bone mass, which increases the risk of developing osteoporosis. Bone mass can be determined by a bone mineral density test (BMD), such as a dual-energy x-ray absorptiometry (DXA). Low bone mass increases the risk of developing osteoporosis and fractures. Osteoporosis can effect any bone in the body, although the most common sites are the wrist, spine and hips. It is credited with more than 1.5 million fractures in both the United States and Canada per year, causing a huge amount of personal suffering and loss of quality of life. (3) (4) This disease also has a high cost to society. The cumulative economic burden of care for fractures due to osteoporosis from 2008-2028 is estimated at $474 billion dollars in the United States alone. (5)
The term, ‘osteoporosis’, describes the condition of the inside of the bones in people who have this disease, where large porous areas develop, weakening the bone structure. Throughout life, bone is a living tissue that maintains a balance through the bone building activity of osteoblasts, with the reabsorptive activity of osteoclasts. When factors such as advancing age cause a change in this balance towards reabsorption, bone mass decreases. After reaching a ‘fracture threshold’, bone that was normally able to withstand a minor stress, such as a fall or blow, becomes subject to break or fracture more easily. Osteoporosis is most often diagnosed in the senior years. However, the most important time to focus on building healthy bone is during the first 3 decades of life. Providing sufficient bone building nutrients, along with weight bearing exercise, may be the best protection against this disease. (6)
There are several risk factors that increase the chance for an individual to develop osteoporosis: family history, female (six to eight times more likely than male) especially post-menopausal, due to decreased estrogen levels, advancing age, Caucasian , low calcium intake, smoking, alcohol consumption, a sedentary lifestyle , (7) and soft drink consumption. (8) (9)
Since many of these contributing factors are self regulated, health care providers can have a direct impact on this health issue by diligently educating clients. Risk of osteoporosis is also directly linked to the use of many prescription and OTC drugs: corticosteroids/steroids, thyroid hormones, anticonvulsants, aluminum containing antacids(ironically often recommended as a calcium source by mainstream physicians), loop diuretics, gonadotropin-releasing hormones, and many others. (10) Wherever possible, health care providers can instruct clients about natural therapies that may be equally effective for specific health conditions, but present a substantially lower risk for interfering with bone density.
Drug therapies for osteoporosis include bisphosphonates, such as alendronate and risedronate. A growing list of concerns is linked to the use of these drugs, including research that suggests a link between the use of these agents and esophageal cancer. (11) A once per month tablet, ibandronate sodium, claims the advantage of greater convenience, but still has a host of possible adverse effects such as esophageal irritation, heartburn, and ulcers. In addition, it is not recommended that women with hypocalcemia take these drugs. Hormone replacement therapy was previously touted as a treatment, and may, in fact be quite useful for decreasing bone loss. However, this benefit decreases if hormone therapy is discontinued. In addition, many women refuse hormone therapy due to other known or perceived adverse effects. (12) (13) Although pharmaceutical agents can be effective, there is an increased interest in non pharmacological prevention and treatment of osteoporosis. (14)
Health care providers can be proactive on this front by supporting the improvement of nutritional status through diet and nutritional supplementation, along with suggestion for an increase in exercise training.
There are several natural interventions that promote increased bone health. These include sufficient consumption of bone supportive nutrients through healthy eating, regular exercise, and nutritional supplements including calcium, magnesium, vitamin D, boron, strontium, soy isoflavones and Vitamin K. Novel supplements such as bone morphogenic proteins are also under investigation. (15)
Exercise
Exercise has an important impact on bone health. Several studies have increased awareness on how exercise can most constructively be used to prevent the development of osteoporosis. (16)
Starting to exercise at a young age is best to achieve long term positive effects, since maximum bone mass is usually achieved during the first third of the life cycle. However, exercise at any age can improve bone health. Weight bearing exercises, including weight training, hiking, climbing stairs and walking, and other exercises that force the bones to work against gravity, are effective at increasing bone mass. (17) Researchers from the Bone & Joint Injury Prevention & Rehabilitation Center at the University of Michigan investigated a host of exercise studies from 1961 to 2009 to determine the kind of exercise that had the greatest impact on bone health and density. They concluded that three factors were most important in predicting the best exercise outcome: Strain magnitude (how much impact the exercise has on the bones and muscles), strain rate (how often maximum vs minimum strain is applied ) and strain frequency ( how often strain occurs in a given amount of time). (18)
A combination of these three factors determines how helpful a given exercise regime is in helping increase bone density. However, there is no consensus about the exact combination of these three factors that is most likely to maximize osteogenic activity. (19) For most individuals, practicing weight bearing exercise three times per week for 12 to 20 minutes is sufficient to increase bone density. Since each joint will respond to the strain load individually, its best to rotate exercise sites, and focus on each one for a limited time period. Continuing to exercise throughout life helps to reduce bone loss and the risk of falls. (20)
Dietary Interventions
The best approach to getting sufficient nutrients to build and maintain strong bones is by consistently making healthy food choices. As we discuss each nutrient below, food sources will be included, along with suggestions for possible supplementation, which is secondary to whole food ingestion.
Calcium
Calcium is the most abundant mineral in the human body. It is well-recognized for its importance in the development of bones and teeth, and has many other functions as well. The ability of calcium supplements to “maintain good bone health and reduce the high risk of osteoporosis later in life.” is one of the few health label claims allowed by the United States FDA. The best food sources of calcium, other than dairy, include whole grains, beans, almonds and other nuts, and dark green leafy vegetables, such as kale. (21) Milk and dairy products contain a substantial amount of calcium; however, it is interesting to note that individuals who avoid dairy due to lactose intolerance do not experience a corresponding increase in osteoporosis. (22) Calcium supplements have been shown in several studies to be effective at slowing bone loss in both peri-menopausal and post menopausal women. (23) A Cochrane Database Review Article (2004), states that “calcium supplements ….. at 500 to 2000 mg per day, are the simplest and least expensive way to prevent bone loss.” (24) A comprehensive literature review published in the British Medical Journal (2010) questioned the commonly held belief in the benefits of using calcium supplements. In this meta-analysis the reviewers concluded that subjects who took a 500 mg/day calcium supplement (without Vitamin D), experienced an increased risk of myocardial infarction, when compared to those who did not take calcium supplements. These results will likely lead to further investigation of current recommendations. (25)
To maintain bone health, 1000- 1500mg/day of calcium (including food sources and supplements) is recommended (varies with age, weight, sex, etc.) by the National Academy of Sciences. (26) Sufficient calcium intake is important in preventing osteoporosis, because if thebody’s stores of calcium is low, calcium will be leached from bones, which can lead to decreased bone mass and the initiation or worsening of osteoporosis. While diet is the ideal source for all nutrients, calcium supplementation is often recommended to ensure that adequate amounts of this important mineral are ingested daily. This can be confusing, due to the many forms of calcium on the market, the differences in dosage levels, absorption rates, delivery forms (ie tablets, vs. liquids), cost, etc. Several studies have shown that calcium citrate is absorbed better than tricalcium phosphate, calcium lactate and calcium carbonate, (the kind of calcium in antacid tablets). (27) Calcium citrate does not tend to cause gastric distress, and has a pleasant taste. One study surmised that calcium formate is better absorbed than either calcium citrate or calcium carbonate. (28) Microcrystalline hydroxyapetite (MH) is a form of calcium that was demonstrated to be more effective at slowing bone loss than calcium carbonate. (29) MH was also shown to support bone density in a randomized double blind 2007 control study. (30) Since calcium is so intimately involved in an array of metabolic reactions, it is not surprising that there is a long list of possible interactions with pharmaceutical drugs. Examples follow: Calcium decreases the absorption of bisphosphonates, (31) levothyroxine (32) , tetracycline and quinolone antibiotics (33) Thiazide can reduce calcium excretion, leading to hypercalcemia, metabolic alkalosis and renal failure. (34) Health care practitioners can assist customers to choose a calcium supplement that best meets their needs.
Magnesium
Magnesium is the second most common mineral in the body (after calcium). Magnesium is important for many metabolic processes, including building bone, formation of ATP, and promoting calcium absorption. Dietary sources of magnesium include nuts, whole grains, dark green vegetables, fish, meat and legumes. Magnesium is often deficient in the Standard American Diet, due to eating a diet low in this nutrient, and soil depletion due to commercial farming practices such as overcroping. (35) Low levels of blood magnesium correlates with low bone density, (36) and several studies have supported the use of oral magnesium supplementation to increase bone density. (37) (38) (39) (40) Even a moderate magnesium deficiency has been documented to cause bone loss in rats. (41) Magnesium deficiency may impair the production of parathyroid hormone and 1,25-dihydroxyvitamin D, which negatively effects bone mineralization. (42) Supplementing with 250-400 mg a day of magnesium is usually recommended. Magnesium glycinate or gluconate are preferable to magnesium oxide, and are less likely to cause loose stools. Adverse effects of magnesium usually occur at higher dosages, and are most often associated with intravenous magnesium. These may include: diarrhea, drowsiness, loss of tendon reflexes, thirst, hypotension, muscle weakness and respiratory and cardiac irregularities. (43) Drug interactions include neuromuscular weakness and possible paralysis when combined with aminoglycoside antibiotics, decreased absorption of biphosphates, tetracycline antibiotics and calcium channel blockers(take at different times). Conversely, many drugs cause hypomagnesemia, including aldesleukin, aminoglycosides and amphotericin-B(common). (44) Magnesium supplementation helps to balance a number of health issues in addition to osteoporosis, such as insomnia, headaches, chronic constipation, restless leg syndrome, anxiety and irritability, and is often the first supplement we recommend in our clinical practice, after implementing a whole food based diet.
Vitamin D
Vitamin D is essential for the formation and maintenance of bone tissue, due to several complex mechanisms, including the regulation of calcium and phosphorous absorption. If Vitamin D levels are low Parathyroid hormone (PTH) increases, and triggers osteoclasts to release calcium into the blood via bone adsorption. If this process continues over time it weakens bone and leads to osteoporosis. In addition, vitamin D stimulates intestinal epithelial cells to synthesize calcium-binding proteins that support the absorption of calcium in the blood. (45)
Vitamin D is called ‘the sunshine vitamin’ because the best source of vitamin D is from sensible sun exposure. Vitamin D is synthesized when sunlight hits the skin and transforms 7-dehydrocholesterol into vitamin D3 (cholecalciferol). D3 is shuttled to the liver where it is converted to 25-hydroxycholecalciferol, which is then transformed into 1,25 dihydrocholecalciferol (calcitriol); 10 times more potent than Vitamin D3. Magnesium and boron act as co-factors in this reaction. Food sources of vitamin D include fish and fish oils. Vitamin D deficiency is now recognized as an epidemic in the United States (46), and is especially common in dark skinned persons, the elderly, people living in northern areas, and anyone who has limited sun exposure. Deficiency can create secondary hyperparathyroidism, leading to a loss of collagen matrix and minerals, which increases the risk of osteoporosis and fractures. Poor bone remodeling due to higher osteoclast vs. osteoblast activity can be due to low levels of vitamin D, reduced synthesis of calcitriol in the kidneys or a lack of calcitrol receptors in target organs (47) Vitamin D is available as a supplement in several forms. Vitamin D 3 ( cholelcalciferol) Vitamin D 2( ergocalciferol) and Alfacalcidol are three common forms. Studies indicate that alfacaldidol has been shown to prevent osteoporosis in women on high dose corticosteroids, (48) as well as increasing muscle power and walking distance in the elderly. (49) A study which compared results using alfacalcidol with ergocalciferol (Vitamin D 2) in elderly women with vertebral fractures, discovered that alfacalcidol has a greater effect than D2 at stimulating calcium absorption by bones. (50) Vitamin D 3 is more effective than Vitamin D 2, and is a better supplement choice for most individuals. (51) An exception would be vegans, who prefer not to use any product that may have been animal sourced, since the starting material for D 3 is fish or lanolin. Mechanisms of action of Vitamin D’s role in building healthy bones includes increasing the number and activity of osteoblasts, (52) reducing the activity of osteoclasts, (53) and normalizing the turnover of bone in osteoporosis. (54)
Vitamin D appears to be most effective as a therapy for osteoporosis when combined with calcium. (55) While 400 IU’s of oral vitamin D (cholecalciferol) is the current RDA, this level of supplementation appears to be insufficient to prevent fractures, while 700 to 800 IU/d appears to reduce the risk of hip and any nonvertebral fractures in both institutionalized and ambulatory elderly persons. (56) Vitamin D is well tolerated at doses of 400 -800 IU. Current studies are moving towards increasing the RDA of Vitamin D, and many health practitioners are already recommending much higher doses. Scandinavian countries are considering ways to increase levels of Vitamin D through both supplementation and the use of UV lights. (57)Vitamin D has a low incidence of adverse effects, although intoxication can result if higher doses are used long term. Symptoms include weakness, nausea, vomiting and poor appetite. Toxicity may be seen when serum 25(OH)D concentration is consistently >200 ng/mL (>500 nmol/L) (58) More problematic are drugs which deplete Vitamin D. These include carbamazepine, (59) cholestyramine and colestipol. (60)
Boron
Boron is ubiquitous throughout the human body with the highest concentrations found in the bones and dental enamel. Although there is currently no RDA, boron appears to be indispensable for healthy bone function, possibly via effects on reducing the excretion and absorption of calcium, magnesium and phosphorus,
( 61) and by affecting signal transmissions across cell membranes by acting indirectly as a proton donor, which influences ion gradients that are involved with cell/cell communication. (62) (63) Boron may be involved in the synthesis of steroidal vitamins and hormones, such as Vitamin D, 17 beta-estradiol and testosterone and inhibits a range of microsomal enzymes which catabolize these steroids, thus delivering a net up-regulatory effect, which could explain its bone building properties. (64) Boron clusters or carboranes have a high binding affinity for steroidal receptors (65) and are being formulated into medications such as specific protease enzyme inhibitors. (66) Boron may be beneficial in the treatment of osteoporosis, especially in the case of vitamin D, magnesium, and potassium deficiency. (67) One study found that boron supplementation as an isolated nutrient was not useful in terms of preventing bone loss. (68) Fruits, vegetables, soybeans and nuts can be rich sources of boron, but the level depends on the soil in which it is grown. A safe daily intake is estimated to be between 1 and 10 mg. Breast cancer patients are often cautioned not to use more than 3 mg a day due to references of boron’s ability to increase endogenous estrogen. (69) Sodium borate and boron chelated with glycinate, aspartate or citrate are the most common forms used in dietary supplements. Toxic effects appear at intakes of about 100 mg. A fatal dose in adults is 15 to 20 g and in children 3 to 6 g. Repeated intakes of small amounts can cause accumulative toxicity, so pulse dosing is recommended, rather than continuous use.
Strontium
The mineral strontium is a powerful agent in the treatment and prevention of osteoporosis. Strontium is a naturally occurring mineral present in water and food. Trace amounts of strontium are found in the human skeleton, where it is adsorbed at the matrix crystal surface of bones. The Spinal Osteoporosis Therapeutic Intervention study is a double-blind, randomized, placebo-controlled trial, which compared two groups of postmenopausal women who already had a diagnosis of osteoporosis. One group was given two grams daily of non-radioactive strontium ranelate , while another group received a placebo. The strontium group illustrated a significant reduction (41%) in the relative risk of experiencing a new vertebral fracture. (70) Other promising studies showed reduced risks for non-vertebral fractures, including hip fractures following the use of strontium. (71) In addition to reducing the risk of fracture, strontium ranelate increased bone mineral density throughout the study, peaking at 3 years, with augmented scores of 8.2% in the femoral neck and 9.8% in the hip. Japanese pharmaceutical researchers have trade named the strontium salt PROTELOS™ and are in phase two drug trials. The mechanism of strontium’s bone strengthening effect is believed to be decreased bone resorption and increased bone formation which increases bone mass, microarchitecture and strength. (72)
In the US, strontium is available as a dietary supplement in the form of strontium citrate. Theoretically, this form may have similar action to strontium ranelate, which has been used in most studies. UC Davis is investigating the use of Sodium Citrate for the prevention of osteoporosis, but the results are not yet available. (73) Most practitioners recommend that strontium should be taken at bedtime, and not at the same time as calcium supplements, since they compete for adsorption into bone matrix.It is important to ensure calcium and vitamin D intakes are adequate when supplementing with strontium. This is underscored by earlier research on animals suggesting that increasing the intake of strontium via diet may de-mineralize bone when calcium is deficient. (74) In rats with chronic kidney failure, strontium has been shown to cause osteomalacia, a condition in which bone is softened due to lack of mineral content. (75) For this reason, it is suggested that people on kidney dialysis should not use strontium supplements.
Isoflavones
Research supports the positive effects of soy isoflavones for reducing the risk of developing osteoporosis. (76) Diets high in soy may decrease bone re-absorption in postmenopausal women. (77) Although ipriflavone, a semi synthetic flavone comparable to genistein and diadzein found in soy foods, was ineffective in restoring bone density in rats, it modulated IGF-I(insulin growth factor I), (78) which is linked to bone mineral density and increased bone remodeling through several mechanisms. (79) IGF-I (Somatomedin C) is currently being measured by holistic health practitioners as one of the parameters to assess overall aging. Ipriflavone yielded positive results on bone mass in elderly women with osteoporosis in human trials at doses of 200 mg per day, (80) and seems particularly beneficial when combined with calcium. (81) Moderate soy consumption (2-4 ounces per day) is likely a reasonable and prudent measure due to scientific validation of its positive effects, combined with a low incidence of adverse reactions. Soy can cause allergic reactions in some individuals, and may inhibit thyroid hormone synthesis. (82) Fermented soy is less likely to cause these adverse effects.
Vitamin K
Vitamin K is a fat soluble vitamin known for its effect in blood clotting, which it accomplishes by regulating the coagulation cascade via its ability to bind calcium ions (Ca2+), among other mechanisms. (83) There are three known vitamin K dependent proteins that have been isolated in bone: MGP (matrix Gla protein), protein S and osteocalcin. One of Vitamin K’s roles in helping to maintain healthy bone mass is linked to its importance in the formation of osteocalcin by osteoblasts. The synthesis of osteocalcin requires both Vitamin D and Vitamin K. There are two naturally occurring forms of vitamin K: Vitamin K 1 (phylloquinone), synthesized by plants, and Vitamin K 2 (Menaquinone-n) synthesized by bacteria. The ‘n’ signifies the number of 5 carbon chains that a specific kind of K 2 contains. Vitamin K 2 is available as both M-4 and M-7 as a dietary supplement. Research supports the use of both Vitamin K 1 and Vitamin K 2 in terms of benefits associated with osteoporosis. Vitamin K 1 supplementation has been shown to support a favorable bone biomarker profile. One study included vitamin K 1, along with Hop rho iso-alpha acids, berberine, vitamin D. The treatment group showed a significant decrease in biomarkers that indicate bone turnover. (84) However, in a double blind study which followed patients who were given 500 mcg of Vitamin K 1 for three years their bone density scores were no better than the placebo group. (85) Patients who undergo transplants have an increased risk for osteoporosis. A randomized, double blind, prospective longitudinal study investigated the effect of a dietary supplement which included vitamin K2 (180 mcg menakinon-7) on bone mass of 94 subjects who were followed for the first year after lung and heart transplantation. The outcome showed a favorable effect on bone mass density of the lumbar spine. (86) Although Vitamin K 2 is currently gaining popularity as the preferred form to use in supplementation, Vitamin K 1 is more cost effective, and therefore may be the better choice for some patients.
Vitamin K is a fat soluble substance; however the body does not store a significant amount at any given time. The need to constantly replenish vitamin K through dietary intake is decreased due to the vitamin K cycle, which allows a small amount that is present to be used by the body several times. Vitamin K deficiency is rare, due to the reuse via the vitamin K cycle, and wide availability in the diet. Vitamin K is found in dark green vegetables such as kale, swiss chard, parsley and spinach, and to some extent in Olive and Soybean oils. Deficiency may occur in those taking anti-coagulant pharmaceutical drugs, or who have difficulty with fat metabolism. People who develop osteoporosis have been documented to have a low dietary intake of vitamin K containing foods, (87) as well as low blood levels of Vitamin K. (88) Health practitioners can emphasize the importance of eating high quality (preferably organic) green vegetables as part of the diet. If supplementation with vitamin K is recommended, common doses include the RDA amount of 65-80 mcg/day.
Bone Morphogenic Proteins
In the early 1960’s, orthopedic surgeon Dr. Marshall Urist discovered a family of proteins that stimulates osteoblasts and cartilage chondrocytes, and named these proteins Bone Morphogenetic Proteins – or BMPs. The impact of Dr. Urist’s contribution to medicine and healthcare was first realized in the 1990’s when commercial bone-protein preparations containing BMP’s and key growth factors were used by orthopedic surgeons for bone healing and spinal fusions. In 2002, the FDA approved select individual BMPs for use in surgical procedures as a more effective way to grow and heal bone. BMP’s account for the major proportion of the osteoinductive potential of bone extracts. (89) BMP’s bind to one of the two types of serine and threonine kinase membrane receptors, and upon binding, initiate an intracellular signaling cascade which modulate the activity of transforming growth factor beta ligands. (90) This in turn leads to the expression of the transcription factorCbfa1 (Runx2), which results in the expression of several proteinscritical for bone formation, ultimately leading to regulation of target genes involved in bone remodeling. (91) BMPs are thought to be key regulators of embryonic skeletogenesis (92), endochondral ossification (93), bone remodeling (94) (95), fracture repair (96), and bone regeneration. (97) Over 20 BMPs family members have been identified. (98) It was once thought that BMP’s could only be applied locally by orthopedic surgeons for a procedure known as “screw and glue” as they attempt to mend a fracture, but recent research in animals suggest that that systemically administered BMP-6 restores the bone inductive capacity, micro-architecture, and quality of the skeleton in osteoporotic rats. Human trials are needed. (99) Somehealth practitioners are now recommending the use of oral BMP’s for osteroporosis and osteopenia at a dosage of 200-1000 mg/day with minimum adverse effects, except for occasional GI upset in some patients.
Health care practitioners can be instrumental in educating their patients to the fact that, with intelligent dietary and lifestyle choices, osteoporosis is largely preventable for most people.
References
- National Osteoporosis Foundation website: http://www.nof.org/osteoporosis/diseasefacts.htm
- Source: http://www.niams.nih.gov/Health_Info/Bone/Bone_Health/bone_mass_measure.asp#e 8/21/2010
- Website: sourced 8/21/2010 http://www.wrongdiagnosis.com/f/fractures/prevalence.htm#incidence_intro
- Sawka AM, Thabane L, Papaioannou A, et. al. Health-related quality of life measurements in elderly Canadians with osteoporosis compared to other chronic medical conditions: a population-based study from the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int. 2005 Aug 18
- Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL, American Academy of Orthopedic Surgeons, February 2008
- Davies JH, Evans BA, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005 Apr;90(4):373-8
- http://www.nof.org/prevention/risk.htm accessed: 8/22/2010
- Wyshak G, Frisch RE. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J Adolescent Health 1994;15:210–5
- Mazariegos-Ramos E, Guerrero-Romero F, Rodríquez-Morán F, et al. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in children: a case-control study. J Pediatr 1995;126:940–2.
- http://www.nof.org/prevention/risk.htm accessed:
8/22/2010
- Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: nested case-control study. BMJ2010;341:c4444.
- Schonberg MA, Davis RB, Wee CC. After the Women’s Health Initiative: decision making and trust of women taking hormone therapy. Womens Health Issues. 2005 Jul-Aug;15(4):187-95
- Selby P.Postmenopausal osteoporosis. Curr Osteoporos Rep. 2004 Sep;2(3):101-6
- Ishikawa-Takata K Ohta T. Nonpharmacological prevention and treatment for osteoporosis. Clin Calcium. 2005 Sep;15(9):1463-6
- Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women : interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35(9):779-830
- Warden SJ, Fuchs RK, Turner CH. Steps for targeting exercise towards the skeleton to increase bone strength. Eura Medicophys. 2004 Sep;40(3):223-32
- Website NIH Osteoporosis and related Bone diseases National Resource Center, accessed 9/2010 http://www.niams.nih.gov/Health_Info/Bone/Bone_Health/Exercise/default.asp#b
- L. Manske, C.R. Lorincz, R.F. Zernicke. Bone Health: Part 2, Physical Activity. Sports Health: A Multidisciplinary Approach July 2009 1:341-346 as listed on http://sportsmedicine.about.com/od/tipsandtricks/a/ExerciseandBones.htm
- Foldhazy Z., Arndt A., et. al., Exercise-induced strain and strain rate in the distal radius. J Bone Joint Surg Br. 2005 Feb;87(2):261-6.
- Sawka AM, Thabane L, Papaioannou A, et. al. Health-related quality of life measurements in elderly Canadians with osteoporosis compared to other chronic medical conditions: a population-based study from the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int. 2005 Aug 18
- Heaney RP, Weaver CM. Calcium absorption from kale. Am J Clin Nutr 1990; 51:656-657.
- Enattah N., Pekkarinen T, Valimaki MJ, et. al., Genetically defined adult-type hypolactasia and self-reported lactose intolerance as risk factors of osteoporosis in Finnish postmenopausal women. Eur J Clin Nutr. 2005Oct;59(10):1105-11
- Di Daniele N, Carbonelli MG, Candeloro N,, et. al. Effect of supplementation of calcium and vitamin D on bone mineral density and bone mineral content in peri- and post-menopause women; a double-blind, randomized, controlled trial. Pharmacol Res. 2004 Dec;50(6):637-41
- Shea B, Wells GA, Cranney A, Zytaruk N, Griffith L, Hamel C, Ortiz Z, Peterson J, Tugwell P, Welch V. Calcium supplementation on bone loss in postmenopausal women. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No.: CD004526. DOI:10.1002/14651858.CD004526.pub3
- Mark J Bolland, Alison Avenell, John A Baron, Andrew Grey, Graeme S MacLennan, Greg D Gamble, Ian R Reid . Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis BMJ 341:doi:10.1136/bmj.c3691 (Published 29 July 2010)
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington DC: National Academy Press, 1997, 108–17
- Heaney RP, Rafferty K, Dowell MS , et. al. Calcium fortification systems differ in bioavailability. J Am Diet Assoc. 2005 May;105(5):807-9
- Hanzlik RP, Fowler SC, Fisher DH.. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J Pharmacol Exp Ther. 2005 Jun;313(3):1217-22
- Straub, D.A. (2007). “Calcium Supplementation in Clinical Practice: A Review of Forms, Doses, and Indications”. NCP- Nutrition in Clinical Practice 22 (3): 286.
- Tucker, L.A.; Nokes, N.; Adams, T. (2007). “Effect of a Dietary Supplement on Hip and Spine BMD: A Randomized, Double-blind, Placebo-controlled Trial: 1515: Board# 5 May 30 2: 00 PM-3: 30 PM”. Medicine & Science in Sports & Exercise 39 (5): S230
- Peters ML, Leonard M, Licata AA. Role of alendronate and risedronate in preventing and treating osteoporosis. Cleve Clin J Med 2001;68:945-51
- Schneyer CR. Calcium carbonate and reduction of levothyroxine efficacy. JAMA 1998;279:750
- Pletz MW, Petzold P, Allen A, et al. Effect of calcium carbonate on bioavailability of orally administered gemifloxacin. Antimicrob Agents Chemother 2003;47:2158-60
- Friedman PA, Bushinsky DA. Diuretic effects on calcium metabolism. Semin Nephrol 1999;19:551-6
- Walsh T., O’Donohoe T., Magnesium deficiency in some crop plants in relation to the level of potassium nutrition. The Journal Of Agricultural Science. (1945), 35 : 254-263 Cambridge University Press online: http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=4532852
- Saito N, Tabata N, Saito S, et. al. Bone mineral density, serum albumin and serum magnesium. J Am Coll Nutr. 2004 Dec;23(6):701S-3S.
- Sojka JE, Weaver CM, Magnesium supplementation and osteoporosis. Nutrition Review 1995 Mar;53(3):71-4.
- Stendig-Lindberg G, Tepper R, Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporsis. Magnes Res 1993;6:155-63.
- H.-P. Dimai, S. Porta, G. Wirnsberger, M., et. al., Daily Oral Magnesium Supplementation Suppresses Bone Turnover in Young Adult Males. The Journal of Clinical Endocrinology & Metabolism (1998) Vol. 83, No. 8 2742-2748
- O. Carpenter, M. C. DeLucia, J. H. Zhang, G. Bejnerowicz, L., et. al. A Randomized Controlled Study of Effects of Dietary Magnesium Oxide Supplementation on Bone Mineral Content in Healthy Girls J. Clin. Endocrinol. Metab., December 1, 2006; 91(12): 4866 – 4872
- Rude RK, Gruber HE, Norton HJ, e.t, al. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone. 2005 Aug;37(2):211-9
- Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem. 2004 Dec;15(12):710-6
- Sourced 9/12/2010 from website: http://www.naturalstandard.com/monographs/monoframeset.asp?monograph=/monographs/herbssupplements/magnesium.asp%3Fprintversion%3Dtrue
- Sabra R, Branch RA. Amphotericin B nephrotoxicity. Drug Saf 1990;5:94-108
- Wasserman RH, Brindak ME, Mayer SA, Fullmer CS Evidence for multiple effects of vitamin D3 on calcium absorption: response of rachitic chicks, with or without partial vitamin D3 repletion, to 1,25-dihydroxyvitamin D3. Proceedings of the National Academy of Sciences of the USA 1982 Dec;79(24):7939-43
- Holick MF. The vitamin d epidemic and its health consequences. J Nutr. 2005 Nov;135(11):2739S-48S
- Schacht E, Richy F, Reginster JY. The therapeutic effects of alfacalcidol on bone strength, muscle metabolism and prevention of falls and fractures. J Musculoskelet Neuronal Interact. 2005 Sep;5(3):273-84
- Reginster JY, Lecart MP, Richy F. Importance of alfacalcidol in clinical conditions characterized by high rate of bone loss. J Rheumatol Suppl. 2005 Sep;76:21-5
- Schacht, E, Richy F, Reginster JY. The therapeutic effects of alfacalcidol on bone strength, muscle metabolism and prevention of falls and fractures. J Musculoskelet Neuronal Interact. 2005 Sep;5(3):273-84
- Francis RM, Boyle IT, Sutdliffe AM, et. al. A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporosis International 1996;6(4):284-90
- Armas LAG, Hollis BW, Heaney RP. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. The Journal of Clinical Endocrinology & Metabolism Vol. 89, No. 11 5387-5391
- Ramzi S., Khory, Weber J, Farach-Carshoh. Vitamin D Metabolites Modulate Osteoblast Activity by Ca+2 Influx-Dependent Nongenomic Pathways . Journal of Nutrition. 125:16995-17035,1995. http://jn.nutrition.org/cgi/reprint/125/6_Suppl/1699S.pdf
- P. D’Amelio, A. Grimaldi, M. A. Cristofaro, M. et. al. Alendronate reduces osteoclast precursors in osteoporosis . Osteoporosis International, Vol 21, No 10. 1741-1750, DOI: 10.1007/s00198-009-1129-1. https://link.springer.com/article/10.1007%2Fs00198-009-1129-1
- Passeri G, Pini G, Troiano L et. al., Low Vitamin D Status, High Bone Turnover, and Bone Fractures in Centenarians. The Journal of Clinical Endocrinology & Metabolism (2003)Vol. 88, No. 11 5109-5115 http://jcem.endojournals.org/cgi/content/full/88/11/5109
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785-95.
- Bischoff-Ferrari HA, Willett WC, Wong JB, et. al., Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005 May 11;293(18):2257-64
- Grant WB, Juzeniene A., Moan JE. Health benefit of increased serum 25(OH)D levels from oral intake and ultraviolet-B irradiance in the Nordic countries. Scandinavian Journal of Public Health. 2010 Sept 3
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008;88:582S-6S
- Collins N, Maher J, Cole M, et al. A prospective study to evaluate the dose of vitamin D required to correct low 25-hydroxyvitamin D levels, calcium, and alkaline phosphatase in patients at risk of developing antiepileptic drug-induced osteomalacia. Q J Med 1991;78:113-22
- Tonstad S, Silverstein M, Aksnes L, Ose L. Low dose colestipol in adolescents with familial hypercholesterolemia. Arch Dis Child 1996;74:157-60
- Nielsen FH, Hunt CD, Mullen LM et al. Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women, FASEB J 1987;1:394-397
- Jessell TM, Kandel ER, Synaptic Transmission: A bi-directional and Self-Modifiable Form of Cell-Cell Communication. Cell, Vol 72/Neuron, Vol 10(Suppl.) 1-30,Jan1993 http://www.cumc.columbia.edu/dept/neurobeh/jessell/Publications/1993PDF/jessell_kandel.pdf )
- Barr RD, Barton SA, Schull WJ. Boron levels in man: preliminary evidence of genetic regulation and some implications for human biology. Med Hypotheses 1996;46:286-289 . https://www.medical-hypotheses.com/article/S0306-9877(96)90257-1/abstract
- Miljkovic N, McCarty MF. Up-regulatory impact of boron on vitamin D function — does it reflect inhibition of 24-hydroxylase? Med Hypotheses. 2004;63(6):1054-6
- Endo Y, Yamamoto K, Kagechika H. Utility of boron clusters for drug design. Relation between estrogen receptor binding affinity and hydrophobicity of phenols bearing various types of carboranyl groups. Bioorg Med Chem Lett. 2003 Nov 17;13(22):4089-92
- Cigler P, Kozisek M, Rezacova P, et. al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc Natl Acad Sci U S A. 2005 Oct 14
- Schaafsma A, de Vries PJ, Saris WH. Delay of natural bone loss by higher intakes of specific minerals and vitamins. Crit Rev Food Sci Nutr. 2001 May;41(4):225-49
- Biquet I, Collette J, Dauphin JF, and et al. Prevention of postmenopausal bone loss by administration of boron. Osteoporos Int 1996;6 Suppl 1:249
- Nielsen FH, Hunt CD, Mullen LM, Hunt JR. Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women. FASEB J 1987 Nov;1(5):394-7
- Reginister JY, Sarlet, N, Lejeune, E, et. al. Strontium ranelate: a new treatment for postmenopausal osteoporosis with a dual mode of action. Curr Osteoporos Rep. 2005 Mar;3(1):30-4.
- Reginister JY, Seeman E, DeVernejoul, MCet al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study J Clin Endocrinol Metab. 2005 May;90(5):2816-22.
- Marie PJ, Felsenberg D, Brandi ML How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporosis International 2010 Sept 2
- http://www.inspire.com/groups/national-osteoporosis-foundation/discussion/strontium-citrate-study-at-uc-davis/
- Grynpas MD, Marie PJ. Effects of strontium on bone quality and quantity in rats. Bone 1990;11:313-19
- Schrooten, I, Cabrera W, Goodman WG, et al. Strontium causes osteomalacia in chronic renal failure in rats. Kidney Int 1998;54:448-56
- Uenishi K. Recommended soy and soy products intake to prevent bone fracture and osteoporosis. Clin Calcium. 2005 Aug;15(8):1393-8
- Harkness LS, Fiedler K, Sehgal AR, et al. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J Womens Health (Larchmt). 2004 Nov;13(9):1000-7.
- Deyhim F, Smith BJ, Soung do Y et al. Ipriflavone modulates IGF-I but is unable to restore bone in rats. Phytother Res. 2005 Feb;19(2):116-20.
- Niu T, Rosen CJ. The insulin-like growth factor-I gene and osteoporosis: A critical appraisal. Gene. 2005 Sep 21
- Passeri M, Biondi M, Costi D et al. Effect of Ipriflavone on bone mass in elderly osteoporotic women. Bone Miner 1992: 19(suppl 1):S57-62
- Gennari C, Agnusdei D, Crepaldi G, et al. Effect of ipriflavone–a synthetic derivative of natural isoflavones–on bone mass loss in the early years after menopause. Menopause. 1998 Spring;5(1):9-15.
72. Persky VW, Turyk ME, Wang L, et al. Effect of soy protein on endogenous hormones in postmenopausal women. J Clin Nutr 2002;75:145-53
83. Brody T. Nutritional Biochemistry. 2nd ed. San Diego: Academic Press; 1999
84. Holick MF, Lamb JJ, Lerman RH, et. Al., Hop rho iso-alpha acids, berberine, vitamin D3 and vitamin K1 favorably impact biomarkers of bone turnover in postmenopausal women in a 14-week trial. J Bone Miner Metab. 2010 May;28(3):342-50. Epub 2009 Dec 19.
85. Booth SL, Dallal G, Shea MK, et al. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 2008;93:1217–23.
86. Forli L, Bollerslev J, Simonsen S, et. Al. Dietary vitamin K2 supplement improves bone status after lung and heart transplantation. Transplantation. 2010 Feb 27;89(4):458-64
87. Tamatani M, Morimoto S, Nakajima M, et al. Decreased circulating levels of vitamin K and 25-hydroxyvitamin D in osteopenic elderly men. Metabolism 1998;47:195–9
88. Hart JP. Circulating vitamin K1 levels in fractured neck of femur. Lancet 1984;ii:283 [letter]
89. Hoffmann A and Gross,G. BMP signaling pathways in cartilage and bone formation. Crit. Rev. Eukaryot. Gene Expr. 11, 23–45. 2001
90. Heldin CH, Miyazono K, ten Dijke P (December 1997). “TGF-beta signalling from cell membrane to nucleus through SMAD proteins”. Nature 390 (6659): 465–71
91. Garimella,R et al. Expression and Synthesis of Bone Morphogenetic Proteins by Osteoclasts: A Possible Path to Anabolic Bone Remodeling. Journal of Histochemistry & Cytochemistry. Volume 56(6): 569–577, 2008
- Hogan BL et al. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594 1996
- Grimsrud et al. , BMP-6 is an autocrine stimulator of chondrocyte differentiation. J Bone Miner Res 14:475–482 1999
- Wozney JM (1992) The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev 32:160–167 1992
- Wozney JM, Rosen V (1998) Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 346:26–37.1998
- Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, Sampath TK, et al. (1998) Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone 22:605–612
- Groeneveld EH, et al. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol 142:9–21. 2000
- Cao, X., and Chen, D.. The BMP signaling and in vivo bone formation. Gene 357, 1–8. & Wozney, J. M. The bone morphogenetic protein family and osteogenesis. Mol. Reprod. Dev. 32, 160–167. (1992)
- Petra Simic et al.Systemically Administered Bone Morphogenetic Protein-6 Restores Bone in Aged Ovariectomized Rats by Increasing Bone Formation and Suppressing Bone Resorption. THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 35, pp. 25509–25521, September 1, 2006
Image Credit:
By BruceBlaus – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=46602308